While more clinical trials are outsourced to CROs/EDC vendors, the needs for ensuring clinical data quality become increasingly important. Sponsor companies with very limited data management resources need an easy-to-use tool to allow clinical data managers to validate existing clinical data, and catch the data issues in the early stage.
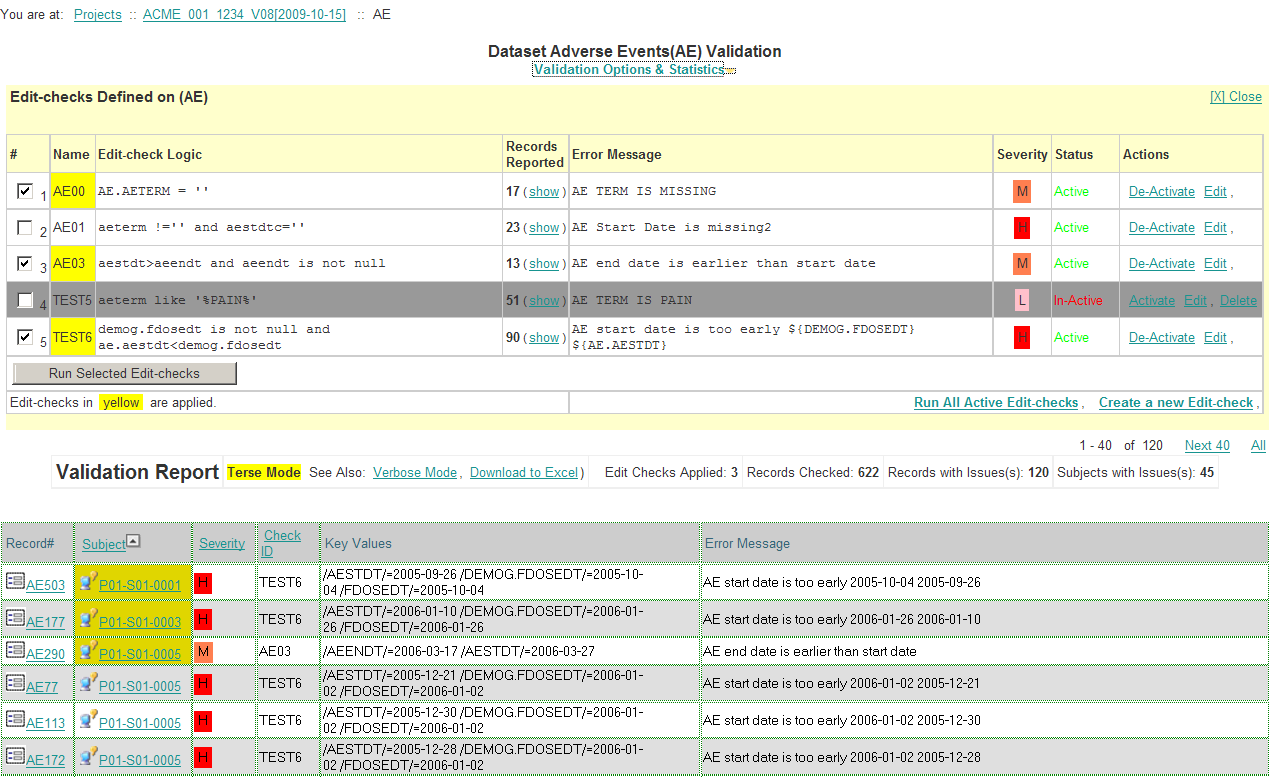
The process of clinical data validation is to apply a set of validation rules to datasets. A validation rule is like an edit check in EDC world, however it can be defined and applied dynamically at any time.
The validation engine is vendor neutral and works with both CDISC and non-CDISC data. No EDC system, SQL, or SAS knowledge is required. The same validation tool can be applied to any clinical studies from any CRO vendors regardless of data capture systems they use.

| Supported Validation Types | Examples |
| Ensure required field and field completeness | |
| Data format compliance | Date of birth, lab data with correct unit |
| Values within designed ranges | Gender (M/F), vital sign valid ranges, etc. |
| In-form cross-field check | AE end date must be later than its start date |
| Cross-form field check | AE start date must be later than the screen date |
| Logic-support cross-field check | If other choice is checked, the "other" value must be set |
| Advanced programmed edit-check/validation | Subjects of protocol deviations/violations based on designated criteria |